Background
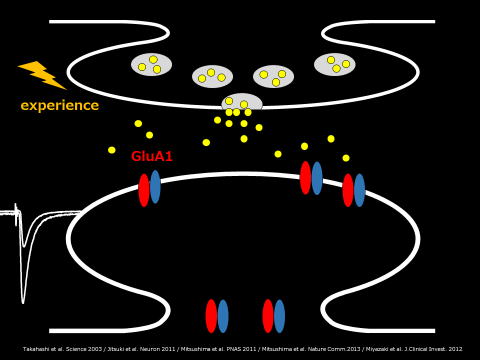
Excitatory synaptic transmission in the central nervous system is mainly mediated by AMPA type glutamate receptors. AMPA receptors form tetrameric heteromers of subunits. Subunits can be classified into two groups based on the structure of their cytoplasmic tail. GluA1, GluA4 and GluA2long (an alternate splice form of GluR2) possess long cytoplasmic tails while GluA2 and 3 have short cytoplasmic tail. There is considerable evidence that synaptic insertion of GluA1-containing AMPA receptors contributes to the synaptic strengthening observed during LTP (Long term potentiation) in vitro and experience in vivo.
Current Research Themes
1. Novel drugs to accelerate the recovery of the motor function with rehabilitation after brain injury (collaboration with FUJIFILM)
Using our in vivo system to evaluate experience-dependent synaptic AMPA receptor delivery, we discovered a novel drug to facilitate experience-dependent synaptic AMPA receptor delivery. This drug accelerates motor function recovery after brain injury (such as stroke) in a training dependent fashion with rodent. We also detected prominent effects with Macaca. We are now preparing for the clinical trial. We hope this will decrease the agony of patients with severe paralysis and minimize the economic burden.
- Hiroki Abe#, Susumu Jitsuki#, Waki Nakajima#, Yumi Murata#, Aoi Jitsuki-Takahashi, Yuki Katsuno, Hirobumi Tada, Akane Sano, Kumiko Suyama, Nobuyuki Mochizuki, Takashi Komori, Hitoshi Masuyama, Tomohiro Okuda, Yoshio Goshima, Noriyuki Higo, Takuya TAKAHASHI.
CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage.
Science 360; 50-57, 2018
2. Development of PET probe to visualize AMPA receptors in human
Recent advance in rodent physiology is noteworthy. The roles of AMPA receptors in the synaptic plasticity, circuit, and behavior have been well elucidated in rodent, and the importance of AMPA receptors in neuronal function has been well established. However, the roles of the AMPA receptors in human cognition and neuronal disease has not been well characterized. To tackle this huge hurdle, we developed PET probe to visualize AMPA receptors in human. We detected specific binding signals with high S/N in rodents and Macaca. Further, we performed first-in human test and obtained potential specific binding signals in living human brain. We started imaging patients of various neuronal disorders such as depression, schizophrenia, addiction, epilepsy, stroke and neurodegenerative diseases. There are a number of compounds which inhibit or enhance AMPA receptors function. However, the clinical trials of these compounds have not been productive. One reason is that these drugs have been tested without examining the distribution of AMPA receptors. By combining our imaging techniques, we believe that the success rate of clinical trials will be increased (eg. AMPA receptors antagonists should be applied to patients with increased AMPA receptors expression). We hope this approach should break out the development of novel drugs targeting at AMPA receptors.
- Miyazaki T, Nakajima W, Hatano M, Shibata Y, Kuroki Y, Arisawa T, Serizawa A, Sano A, Kogami SYamanoue T, Kimura K, Hirata Y, Takada Y, Ishiwata Y, Sonoda M, Tokunaga M, Seki C, Nagai Y,Minamimoto T, Kawamura K, M.R. Zhang, Ikegaya N, Iwasaki M, Kunii N,Kimura Y, Yamashita F, Taguri M, Mimura M, Yuzaki M, Kato H, Higuchi M, Uchida H, Takahashi T
Visualization of AMPA receptors in living human brain with positron emission tomography.
Nature Medicine. 26;281-288, 2020 - Mai Hatano, Tomoyuki Miyazaki*, Yoshinobu Ishiwata, Waki Nakajima, Tetsu Arisawa, Yoko Kuroki, Ayako Kobayashi, YuukiTakada, Matsuyoshi Ogawa, Kazunori Kawamura, Ming‑Rong Zhang, Makoto Higuchi, MasatakaTaguri, Yasuyuki Kimura, Takuya Takahashi
Biodistribution and radiation dosimetry of the positron emission tomography probe for AMPA receptor, [11C]K‑2, in healthy human subjects
Scientifc Reports 11; 1598, 2021 - Tetsu Arisawa, Tomoyuki Miyazaki, Wataru Ota, Akane Sano, Kumiko Suyama, Yuuki Takada, Takuya Takahashi
[11C]K-2 image with positron emission tomography represents cell surface AMPA receptors
Neuroscience Research 173; 106-113, 2021 - Tetsu Arisawa, Kimito Kimura, Tomoyuki Miyazaki, Yuuki Takada, Waki Nakajima, Wataru Ota, Sadamitsu Ichijo, Akane Sano, Yuuka Hirao , Jun-Ichi Kurita, Yoshifumi Nishimura, Takuya Takahashi
Synthesis of [ 18 F] fluorine-labeled K-2 derivatives as radiotracers for AMPA receptors
Nuclear Medicine and Biology 110-111; 47-58, 2022
Previous Research Theme
1. Experience-driven synaptic AMPA receptor delivery
I have previously found that whisker experience drives AMPA receptors into synapses of the developing rat barrel cortex. This is the first evidence of experience-driven synaptic AMPA receptor delivery in vivo. This research is the basis of our system to evaluate synaptic AMPA receptor delivery in vivo.
- Takahashi T., Svoboda K., Malinow R.
Experience strengthening transmission by driving AMPA receptors into synapses.
Science 299; 1585-1588, 2003
2. Cross-modal plasticity
Loss of a sensory input can cause improved function of other intact sensory systems. We found that visual deprivation induced the elevated function of whisker-barrel system of juvenile rats. Synaptic AMPA receptor delivery is facilitated in the barrel cortex of juvenile rats with visual deprivation via increased serotonin secretion. Since this research proves that compensation-induced enhancement of intact function after loss of functions accompanies with the facilitation of AMPA receptors, it is the basis of the development of the compound to accelerate the rehabilitation after stroke.
- Jitsuki S, Takemoto K, Kawasaki T, Tada T, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB,Kessels HW,and Takahashi T.
Serotonin mediates cross-modal reorganization of cortical circuits.
Neuron 69, 780-792, 2011. - Nakajima W, Jitsuki S, Sano A, Takahashi T.
Sustained Enhancement of Lateral Inhibitory Circuit Maintains Cross Modal Cortical Reorganization.
PLoS One, 11(2):e0149068, 2016, Feb 10.
3. Neonatal social isolation disrupts synaptic AMPA receptor delivery
Stressful events during early childhood can induce a malfunctioning of emotional and cognitive behaviors later in life. We found that neonatal social isolation disrupts experience-driven synaptic AMPA receptor delivery in rodent. This research led us to the development of PET probe for AMPA receptors to visualize AMPA receptors in human.
- Miyazaki T, Takase K, Nakajima W, Tada H, Ohya D, Sano A, Goto T, Hirase H, Malinow R, Takahashi T.
Disrupted cortical function Underlies behavior dysfunction due to social isolation.
Clin Invest.,122(7), 2690-701,2012. - Miyazaki T, Kunii S, Jitsuki S, Sano A, Kuroiwa Y, Takahashi T.
Social isolation perturbs experience-driven synaptic glutamate receptor subunit 4 delivery in the developing rat barrel cortex.
Eur J Neurosci., 37(10):1602-9, 2013. - Tada H,Miyazaki T,Takemoto K,Takase K,Jitsuki S,Nakajima W,Koide M,Yamamoto N,Komiya K, Suyamaa K,Sano A,Taguchi A,and Takahashi T.
Neonatal isolation augments social dominance by altering actin dynamics in the medialprefrontal cortex.
PNAS, E7097-E7105, 1606351113, 2016.10
4. Hippocampus dependent learning drives AMPA receptors into CA3-CA1 hippocampal synapses
We have found that hippocampus dependent contextual fear learning drives AMPA receptors into CA3-CA1 hippocampal synapses via acetylcholine elevation.
- Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T.
Contaxtual learning requires synaptic AMPA receptor delivery in the hippocampus.
Proc Natl Acad Sci USA., 108(30),12503-12508, 2011. - Mitsushima D, Sano A, Takahashi T.
Cholinergic trigger drives learning-induced plasticity at hippocampal synapses.
Nature Communications, Vol 4, Article number 2760,2013. - Takemoto K,Iwanari H,Tada H,Suyama K,Sano A,Nagai T,Hamakubo T,and Takahashi T.
Optical inactivation of synaptic AMPA receptors erases fear memory.
Nature Biotechnology, 35(1);38-47, 2017 (*co-corresponding author)




